Welcome to CM Online Store!
Antibody Drug Conjugate Kits!
AqT bioconjugates coming soon!
The most common functional groups targeted for bioconjugation on a protein are hydroxyl (present on Thr, Ser, and the phenolic group in Tyr), carboxylic acid (present at the C-terminus and on Asp and Glu), sulfhydryl (present on Cys), and amine (present at the N-terminus and Lys). Histidine's imidazolyl nitrogen and arginine's guanidinyl groups can also be targeted, but are less commonly used. Most proteins have multiple copies of the same amino acids, and conjugation chemistry targeting the functional groups of natural amino acids can result in heterogeneous bioconjugates.
To specifically label a protein site, an orthogonal functional group can be introduced into the system. So far, the most popular approach uses Cys incorporation by mutagenesis. At CellMosaic, we are experts in dealing with Cys mutant proteins and labeling them with different molecules. Alternatively, if the protein is glycosylated, it can be site-specifically labeled at its glycosylation site. In general, the hydroxyl groups on adjacent carbon atoms of carbohydrates are oxidized to generate formyl groups. At CellMosaic, we have different linkers and molecules that can be synthesized to specifically react with the formyl group under very mild conditions. The less controllable approach for obtaining homogeneous protein conjugates is through manipulation of the reaction conditions in combination with the purification method(s).
At CellMosaic, we carry some labeled proteins, please click here to check some of our products.
We also carry Personalized Conjugation Kits (PerKit™) for protein labeling and conjugation, please click here to check our PerKit™ enzyme labeling and conjugation kits.
For large scale or project beyond the scope of PerKit™ configuration, please contact us for a quote.
Selected examples:
At CellMosaic, we offer custom synthesis of natural peptides, as well as modified peptides such as peptidomimetics, peptides with orthogonal functional groups, fully protected peptides, chimeric peptides, fluorescent or biotin-labeled peptides, peptide–oligo conjugates, and peptide-antibody/protein/enzyme conjugates. We use peptide synthesizer for milligram to gram scale synthesis. In order to obtain the highest quality, all of our peptides are synthesized using the most efficient HATU or HCTU coupling reagent, in contrast to the less efficient HBTU generally used by other peptide houses to reduce costs. We use an Fmoc peptide protocol with modifications deemed necessary to make the highest quality racemization-free peptide.
At CellMosaic, we carry some peptides and modified peptides, please click here to check some of our products.
We also carry Personalized Conjugation Kits (PerKit™) for peptide labeling and conjugation, please click here to check our PerKit™ product line.
For large scale or project beyond the scope of PerKit™ configuration, please contact us for a quote.
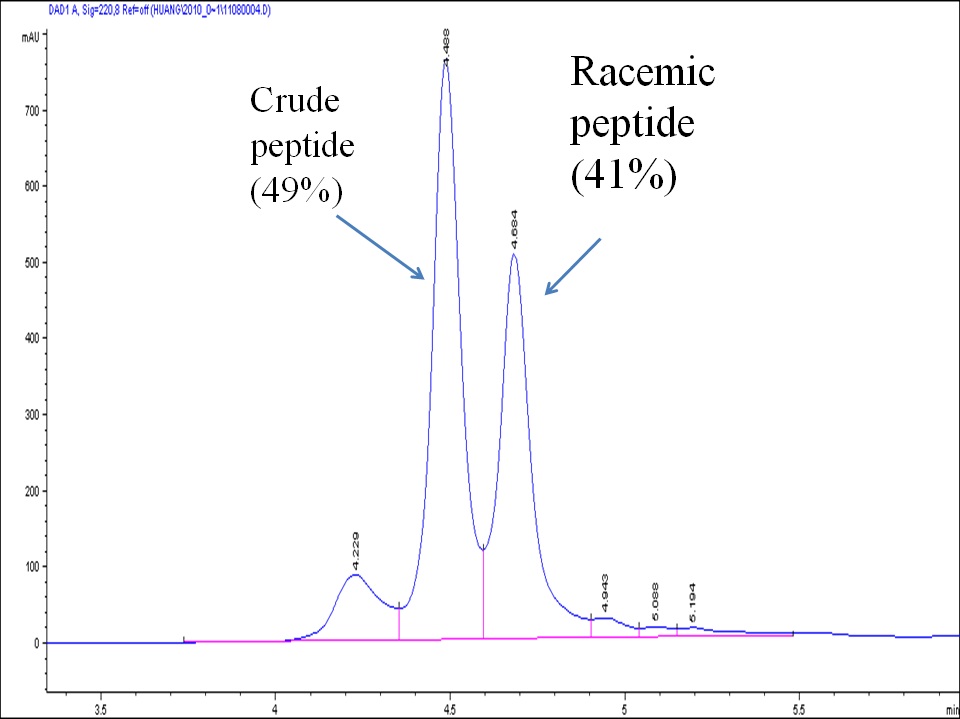
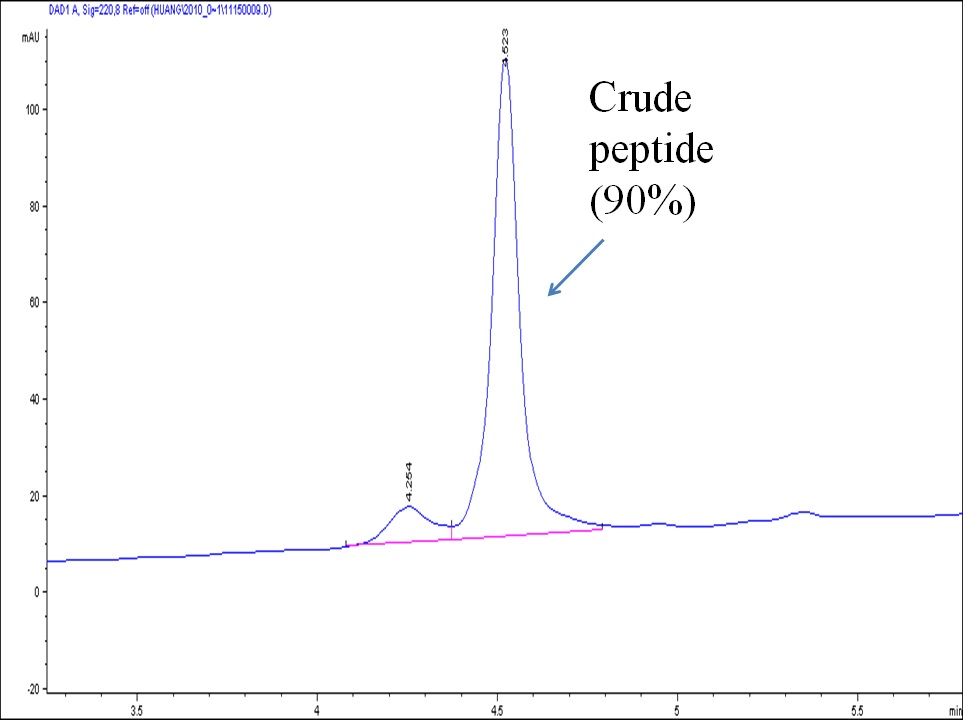
Cysteine has a free thiol group that can be specifically modified or conjugated with other molecules through well-established thiol chemistry; for example, by reacting with another thiol compound to form a cleavable disulfide bonded conjugate, or by reacting with a maleimide or an alkyl halide compound to form a stable thiol ether-bonded conjugate. N-terminal Cys-containing peptides have been used frequently for coupling with an immunogen carrier, such as KLH or BSA, for immunization purposes. Racemization of Cys residues is known to occur during standard HBTU and HATU coupling. This racemization can lead up to 40% byproduct and, in many cases, the racemized products are difficult to purify using standard reverse phase HPLC. To minimize the racemization of Cys residues (and histidine residues) during peptide synthesis, we intervene and perform manual couplings for all Cys and His residues. The outcome of such processes is high quality Cys or His-containing peptides. For peptides containing several Cys residues, we can selectively protect and deprotect Cys for labeling and conjugation. We can also selectively manage inter- or intramolecular disulfide bonds using orthogonal protecting groups for Cys.
Below is an example of a Cys-containing peptide synthesized using the standard automatic procedure (left) vs. manual intervention and special coupling (right)
.
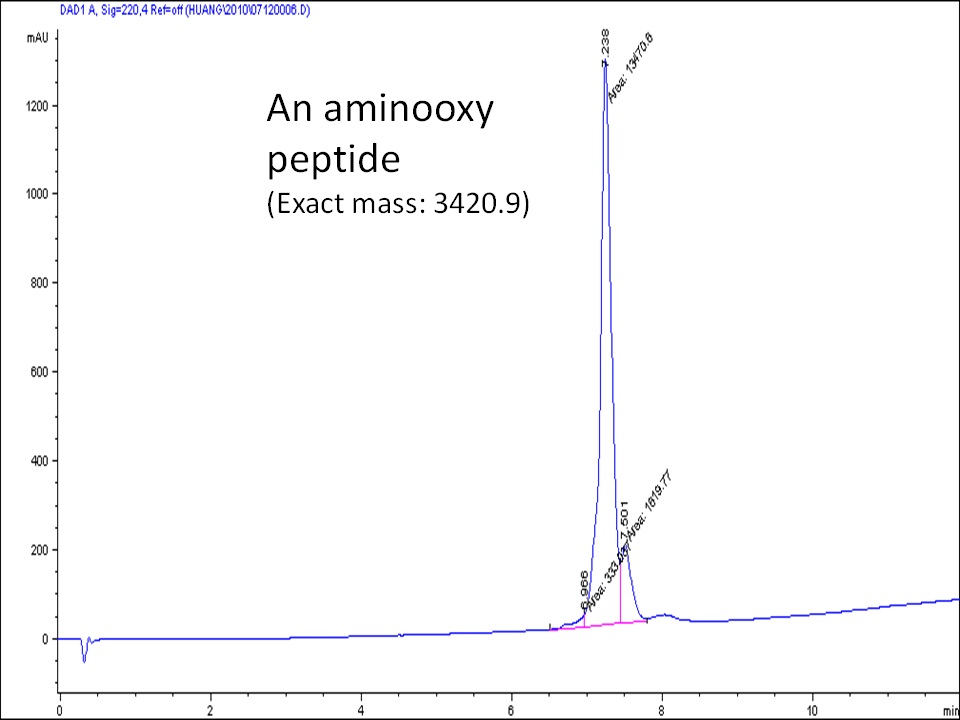
Aminooxy (-ONH2) is known to react quantitatively with ketone or aldehyde-containing compounds under very mild near physiological conditions. The resulting oxime compound is stable for most biological applications. The oxime bond can be further reduced to a more stable imine bond. Despite their usefulness, aminooxy peptides are not easy to make. Commercially available Fmoc protected aminooxy amino acid analogs suffer from double acylation during peptide synthesis. However, using the di-Boc protected aminooxy amino acid results in very poor yield of peptide during TFA cleavage because of acidic decomposition. At CellMosaic, we address this issue by synthesizing a special aminooxy monomer for peptide synthesis and using a clean cleavage cocktail.
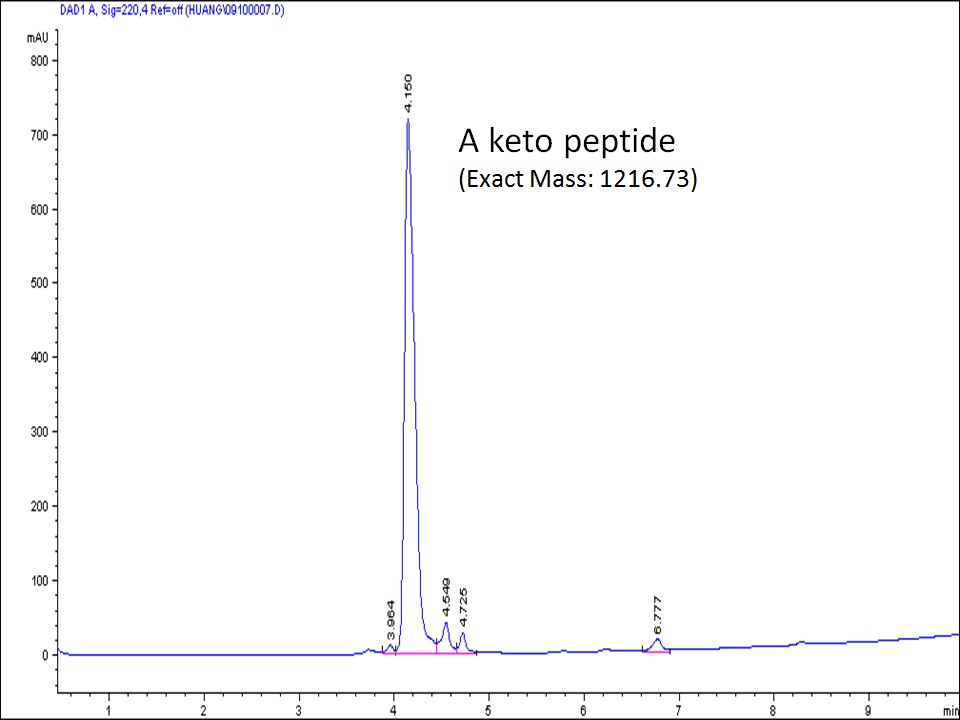
Keto peptides have frequently been synthesized at CellMosaic. Keto peptides can react with any aminooxy or hydrazine-containing compound.
Peptides can be selectively labeled or conjugated through the C-terminal carboxylic acid. To perform such a conjugation for peptides containing few internal Asp and Glu residues and other functional groups, such as free amine and thiol, the peptide needs to be fully protected during modification and conjugation. Usually, trityl chloride or Rink acid resin can be used for synthesizing such fully protected peptide acids. The fully protected peptide acid can then react with any molecule of interest in solution.
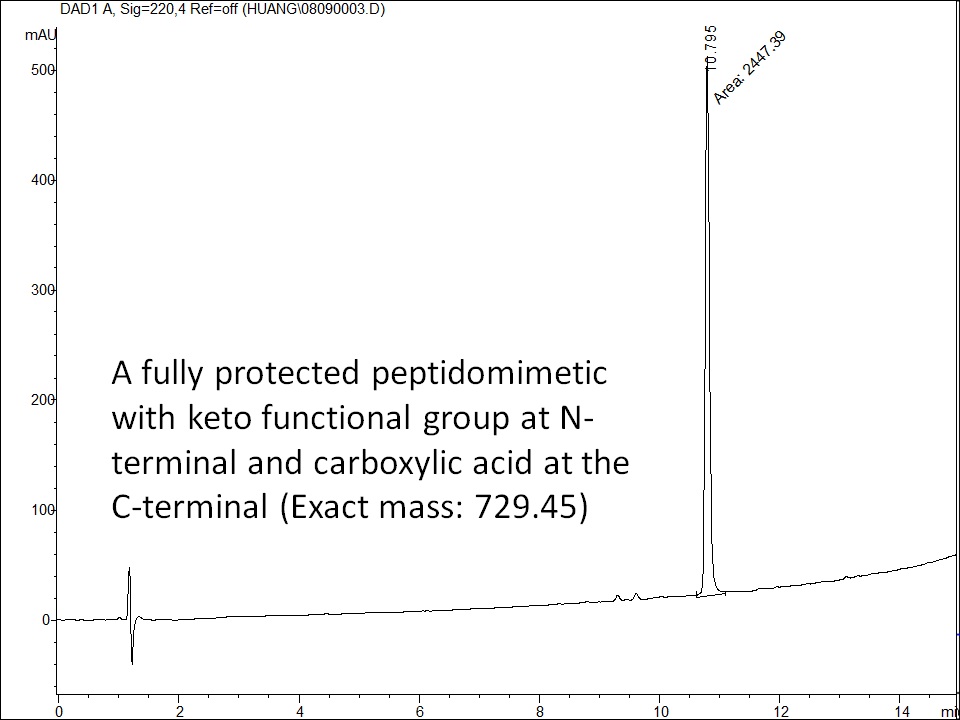
Selected Examples: