Introducing Functional Groups
Examples of biopolymer modification to introduce functional groups for labeling and conjugation.
Aldehyde through Amine
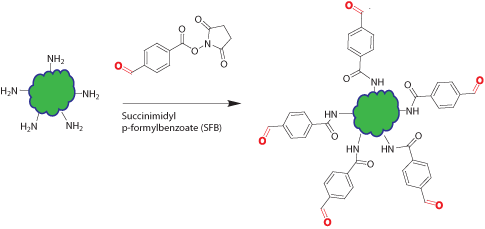
Native proteins, oligos, and peptides have no aldehyde functional group. Introduction of aldehyde groups into these biopolymers allows them to react with other molecules specifically at the aldehyde site through reductive amination, oxime, and hydrazone formation.
This modification introduces aldehyde groups on the surface amine (Lys and N-terminal amine) of the peptide/protein or amino-modified oligo through amine reactive reagent: succinimidyl p-formylbenzoate (SFB). Usually multiple aldehyde groups are introduced onto the biopolymer depending on the population of surface amine functional groups on the biopolymer and the extent of the reaction.
References:
- Kraehenbuhl, J. P.; Galardy, R. E.; Jamieson, J. D. Preparation and characterization of an immuno-electron microscope tracer consisting of a heme-octapeptide coupled to Fab. J. Exp. Med. 1974 Jan 1; 139(1):208-23. PMID: 4357686
- Galardy, R. E.; Stafford, S. S.; Schaefer, M. L.; Ho, H.; La Vorgna, K. A.; Jamieson, J. D. Biologically active derivatives of angiotensin for labeling cellular receptors. J. Med. Chem. 1978 Dec; 21(12):1279-83. PMID: 214560
Aldehyde through Diol
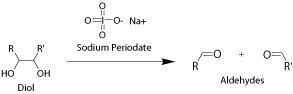
Some native proteins and antibodies contain carbohydrate modifications. Aldhyde groups can be introduced to the carbohydrates through periodate oxidation. Only one or few aldehyde groups are introduced onto the biopolymer depending on the nature of biopolymer and the extent of the oxidation. This method allows site-specific modification and conjugation.
References:
- Bobbit, J. M. Periodate oxidation of carbohydrates. Adv. Carbohydr. Chem. 1956; 48(11):1-41. PMID: 13469627
- Rothfus, J. A.; Smith, E. L. Glycopeptides. IV. The periodate oxidation of glycopeptides from human gamma-globulin. J. Biol. Chem. 1963; 238(4):1402-10. PMID: 13975369
- Lotan, R.; Debray, H.; Cacan, M.; Cacan, R.; Sharons, N. Labeling of soybean agglutinin by oxidation with sodium periodate followed by reduction with sodium [3-H]borohydride. J. Biol. Chem. 1975; 250(5):1955-7. PMID: 163260
- Van Lenten, L.; Ashwell, G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J. Biol. Chem. 1971; 246(6):1889-94. PMID: 4323238
- Wilchek, M.; Bayer, E. A. Labeling glycoconjugates with hydrazide reagents. Methods Enzymol. 1987; 138:429-42. PMID: 3110546
Thiol through Traut's Reagent
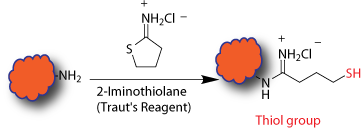
This is an intermediate step which introduces thiol group(s) to a biopolymer and prepares it for conjugation through sulfhydryl groups. The modification is generally done right before the conjugation or labeling, however, the modified biopolymer can be stored in the presence of reducing agents. Basic chemistry invovles reacting amines with 2-Iminothiolane (Traut's reagent) generating sulfhydryl groups in a biopolymer. Multiple sulfhydryl groups are introduced onto the biopolymer depending on the available amine groups on the biopolymer and the extent of the reaction.
References:
- Traut, R. R.; Bollen, A.; Sun, T. T.; Hershey, J. W.; Sundberg, J.; Pierce, L. R.; Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973 Aug 14;12(17):3266-73. PMID: 4581787
- Jue, R.; Lambert, J. M.; Pierce, L. R.; Traut, R. R. Addition of sulfhydryl groups to Escherichia coli ribosomes by protein modification with 2-iminothiolane (methyl 4-mercaptobutyrimidate). Biochemistry. 1978 Dec 12;17(25):5399-406. PMID: 365229
- Lambert, J.M.; Jue R.; Traut, R. R. Disulfide cross-linking of Escherichia coli ribosomal proteins with 2-iminothiolane (methyl 4-mercaptobutyrimidate): evidence that the cross-linked protein pairs are formed in the intact ribosomal subunit. Biochemistry. 1978 Dec 12; 17(25):5406-16. PMID: 365230
Thiol through SATA
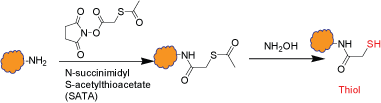
This is an intermediate step which introduces thiol group(s) to a biopolymer through the reaction of amines with N-succinimidyl S-acetylthioacetate (SATA). The modified biopolymer contains a protected sulfhydryl group and can be stored. An extra step is required for removing the thiol protection right before labeling or conjugation. Multiple sulfhydryl groups are introduced onto the biopolymer depending on the available amine functional groups on the biopolymer and the extent of the reaction. The removal of thiol protection doesn't require disulfide reducing reagents.
References:
- Duncan, R. J.; Weston, P. D.; Wrigglesworth, R. A new reagent which may be used to introduce sulfhydryl groups into proteins, and its use in the preparation of conjugates for immunoassay. Anal. Biochem.1983 Jul 1;132(1):68-73. PMID: 6353995
- Derksen, J. T. P.; Scherphof, G. L. An improved method for the covalent coupling of proteins to liposomes. Biochimica et Biophysical Acta - Biomembranes 1985 Mar 28; 814(1):151-155.
Disulfide Bond Reduction
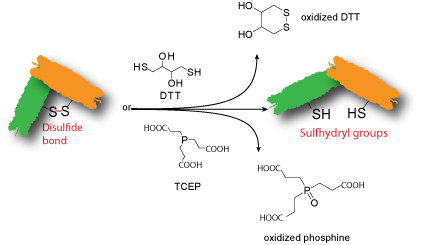
Some biopolymers contain disulfide bonds. If disulfide bonds are not vital to the biopolymer's activity, these disulfide bonds can be cleaved to generate free sulfhydryl (thiol) groups using Cleland's reagent dithiothreitol (DTT) or Tris(2-carboxyethyl)phosphine (TCEP) for further modification.Single or few sulfhydryl groups are introduced onto the biopolymer depending on the number of disulfide bonds present on the biopolymer and the extent of the reaction. The modified biopolymer contains a free sulfhydryl that has a short storage life and should be used immediately for the next reaction.
References:
- Cleland, W. W.; Dithiothreitol, a New Protective Reagent for SH Groups. Biochemistry. 1964, 3, 480-2. PMID: 14192894
- Burns, J A.; Butler, J. C.; Moran, J.; Whitesides, G. M. Selective Reduction of Disulfides by Tris(2-carboxyethyl)phosphine. J. Org. Chem. 1991, 56, 2648-50.
