Product Description
| Download Documents | |
| Product Sheet | |
| SDS | COA_Lot#NB0350512 |
CellMosaic’s Phenyldiazirine sxLink™ is a proprietary photo-crosslinking reagent developed by CellMosaic® for studying biomolecular interactions.
This reagent combines a highly efficient, carbene-generating phenyldiazirine group with convenient oxime-based reversible chemical crosslinking and the super-hydrophilic AqT® linker. These structural features make this reagent highly advantageous compared to traditional photo-crosslinking reagents.
- Trifluoromethyl phenyldiazirine (TFMP) group: TFMP photolyzes around 360 nm, at which photodamage to biomolecules is minimized. The generated carbene inserts C–H bonds into the neighboring biomolecular partner within picoseconds. Because the electron-withdrawing trifluoromethyl group confers stability on the intermediate diazo-isomer, no side products are detected under normal labeling conditions.
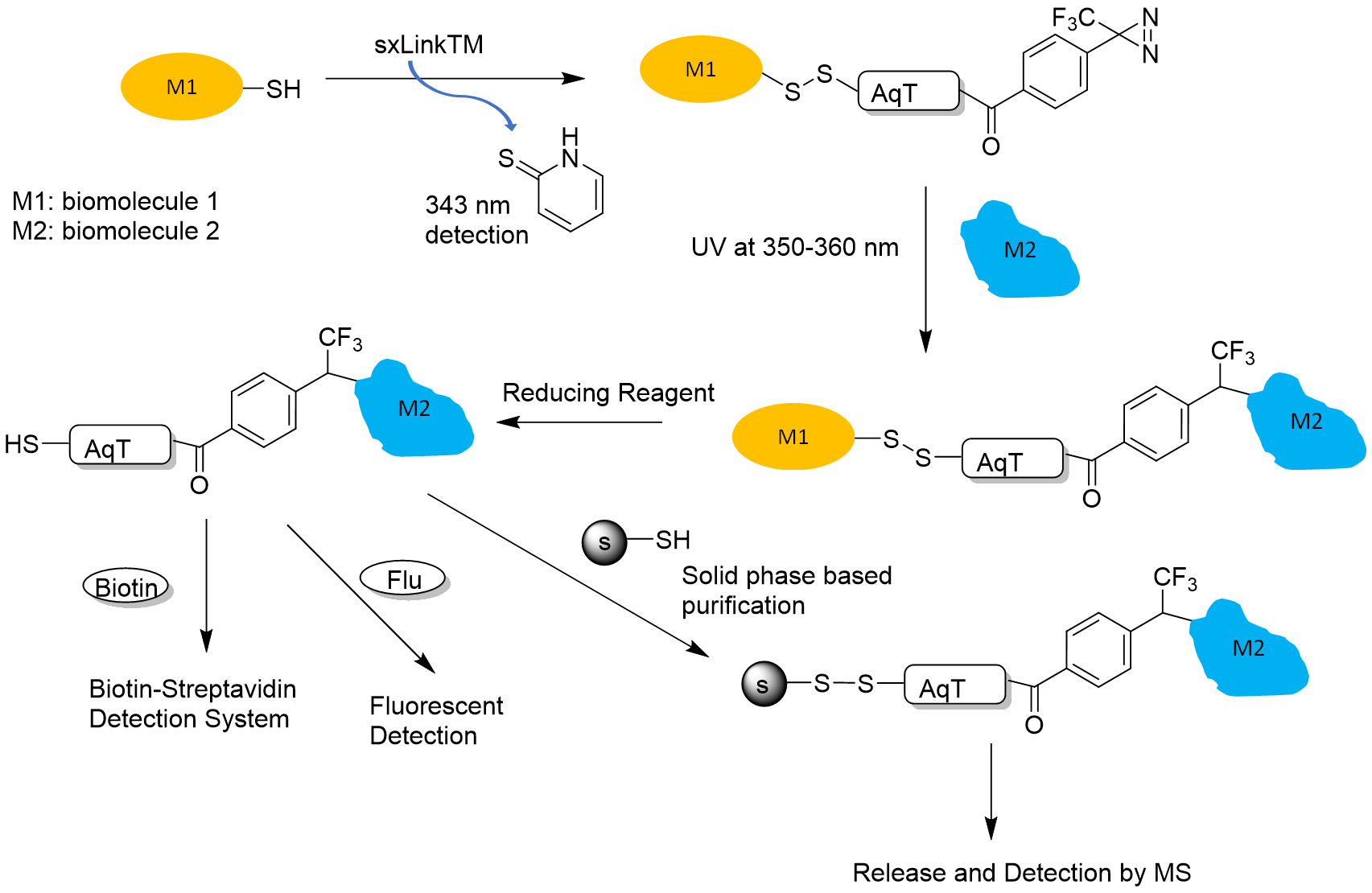
- Reversible disulfide for chemical crosslinking of any biomolecule containing a free thiol. Figure 1 shows a workflow for how a reversible sxLink™ can be used to crosslink the interacting biomolecule partner and identify the crosslinking site or detect the interaction partner.
- Hydrophilic AqT® linkers: TFMP group is highly hydrophobic, biomolecules labeled with a TFMP using a traditional ethylene and ethylene glycol-type linker tend to aggregate and destabilize the labeled protein. The AqT® linker greatly enhances the hydrophilicity and water solubility of sxLink™ (2.2 mg/mL). It also improves biocompatibility and reduces non-specific hydrophobic interactions with other biomolecules, allowing high loading of phenyldiazirine groups.
The product is sold as a lyophilized powder in either 1 vial of 250 µg (Cat# CM81401-250UG) or 4 vials of 250 µg (Cat# CM81401-4x250UG). One vial of dissolution buffer is included in each configuration.
Chemical Information
- Chemical name: Phenyldiazirine sxLink™
- Chemical formula: N/A
- Molecular weight: 633.66 Da CAS number: N/A
Specification
- Physical appearance: white lyophilized powder in a 1.5 mL centrifuge tube
- Storage temp.: -20 °C
- Purity: ≥ 95 % by HPLC
Application of the Product
- Labeling biomolecules containing free thiol groups
- Studying dynamic biomolecule interactions via photo-crosslinking (e.g., studying biological complexes and networks, protein-protein interactions, protein-DNA interactions, and small ligand-protein interactions)
Key Features of the Product
- More hydrophilic than traditional photo-crosslinking reagent, soluble, and biocompatible
- Enable high loading with minimized aggregation
- Reversible linkage
- Includes an efficient carbene-generating photo-crosslinking group
Figure 1. sxLink™ workflow using water-soluble Phenyldiazirine sxLink™
References
Protein crosslinking reviews: a) Brunner, J. (1993) Annu. Rev. Biochem. 62, 485−514. b) Freedman, R. B. (1979) Trends Biochem. Sci. 193–197. c) Herrmann, J. M., Westermann, B., Neupert, W. (2001) Methods Cell Biol. 65, 217–230. d) Fancy, D. A. (2000) Curr. Opin. Chem. Biol. 4, 28–32. e) Fasold, H., Klappenberger, J., Meyer, C., Remold, H. (1971) Angew. Chem. Internat. Edit. 10, 795–801. f) Bayley, H. Photogenerated Reagents in Biochemistry and Molecular Biology, Vol. 12. Elsevier, Amsterdam, Neth, 1983.
3-Trifluoromethyl-3-phenyldiazirine reference: Brunner, J., Senn, H. & Richards, F. M. (1980) J. Bio. Chem. 255, 3313−3318.
Thiol cleavable photo-crosslinking reagents for studying rhodopsin and transducing interactions: a) Resek, J. F., Bhattacharya, S. & Khorana, H. G. (1993) J. Org. Chem. 58, 7598−7601. b) Resek, J. F., Farrens, D. & Khorana, H. G. (1994) Proc. Natl. Acad. Sci. USA 91, 7643−7647. c) Cai, K, Itoh, Y. & Khorana, H. G. (2001) Proc. Natl. Acad. Sci. USA 98, 4877−4882. d) Huang Y, Khorana HG. (2003) Mapping of Contact Sites in Interaction between Transducin and Light-Activated Rhodopsin. Presented at 17th Symposium of the Protein Society, July 26–30, Boston, Massachusetts.
Frequently Asked Questions:
If you can’t find the answer you’re looking for or need information on general topics, please visit the main Frequently Asked Questions (FAQs) section.