There are no products listed under this category.
Welcome to CM Online Store!
Antibody Drug Conjugate Kits!
AqT bioconjugates coming soon!
 CellMosaic® has extensive experience and expertise in preparing peptide- or peptidomimetic-based drug conjugates. We support customers by developing peptide labeling and conjugation strategies, synthesizing the modified peptide, performing the drug conjugation, and fully characterizing the resulting peptide–drug conjugates. For information regarding peptide synthesis and modification at CellMosaic, please click here.
CellMosaic® has extensive experience and expertise in preparing peptide- or peptidomimetic-based drug conjugates. We support customers by developing peptide labeling and conjugation strategies, synthesizing the modified peptide, performing the drug conjugation, and fully characterizing the resulting peptide–drug conjugates. For information regarding peptide synthesis and modification at CellMosaic, please click here.
Drugs can be conjugated at any position on the peptide—C-terminus, N-terminus, or internal residues. In most cases, a single, pure bioconjugate can be obtained. CellMosaic's peptide–drug conjugation process can be easily tailored to meet specific project requirements, offering a fast and efficient solution for developing peptide-based conjugates.
Advantages of CellMosaic's peptide synthesis and conjugation strategy:
Case Studies:
1. Developing a site-specific single-labeled peptide-polymyxin conjugate for a biotech company at CellMosaic® (shown below).
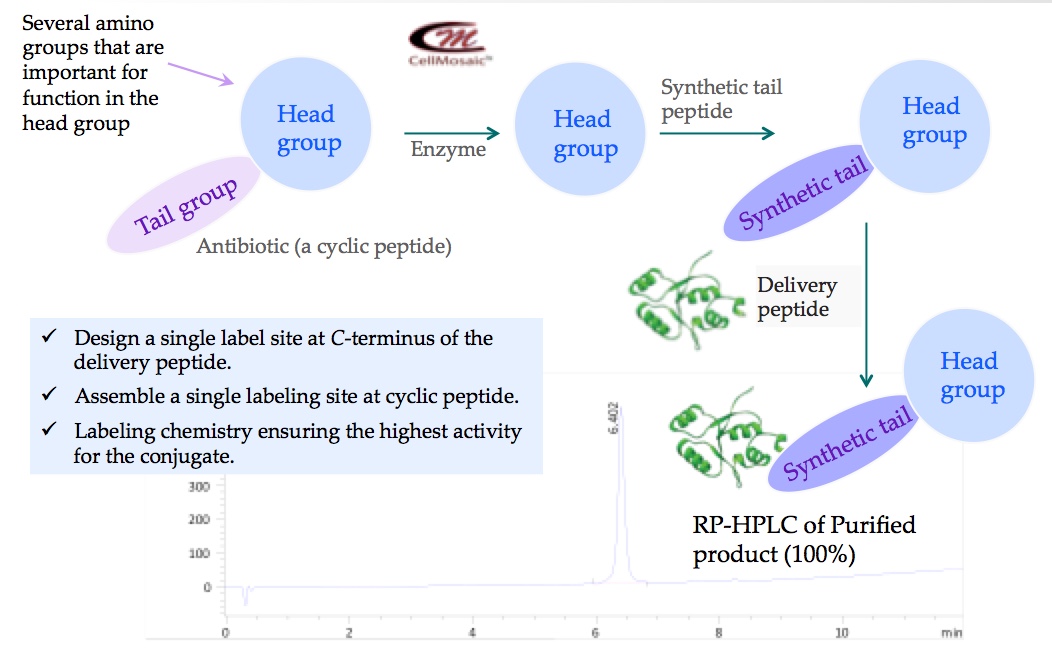
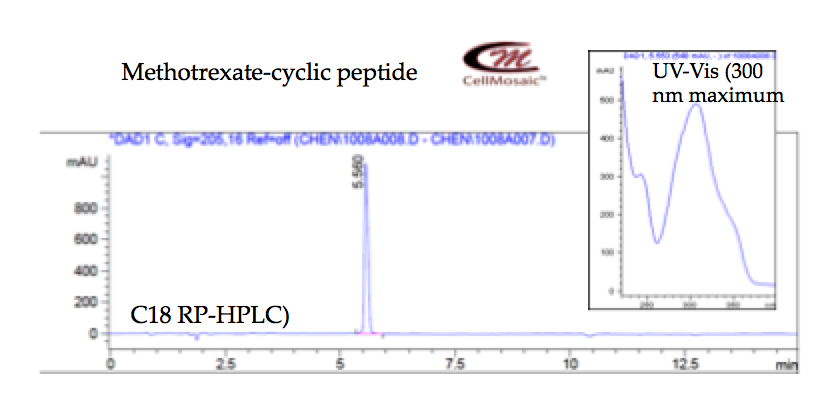
2. Developing strategies for synthesizing cyclic peptide and its conjugate with methotrexate for academic researchers at CellMosaic® (shown below).
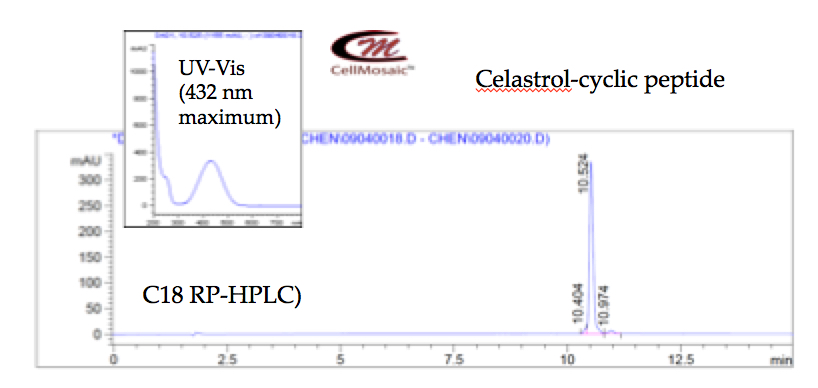
3. Developing strategies for synthesizing cyclic peptide and its conjugate with celastrol for academic researchers at CellMosaic® (shown below).
There are no products listed under this category.