Product Description
Proteins/enzymes/antibodies in their native form usually contain large numbers of surface amine groups (Lys or N-terminal amines). Those surface amine groups can easily react with an acylating reagent, such as NHS-ester or isothiocyanate activated small molecule, to form a stable amide bond or equivalent. The products obtained usually contain multiple labels per molecule. Depending on the nature of the small molecules, total pI of the protein/enzyme/antibody may be altered after labeling.
CellMosaic® has Personalized Conjugation Kits (PerKit™) for protein/enzyme/antibody labeling and conjugation, please click here to learn our PerKit™ product line.
For large scale or project beyond the scope of PerKit™ configuration, please contact us for a quote.
Examples (see images):
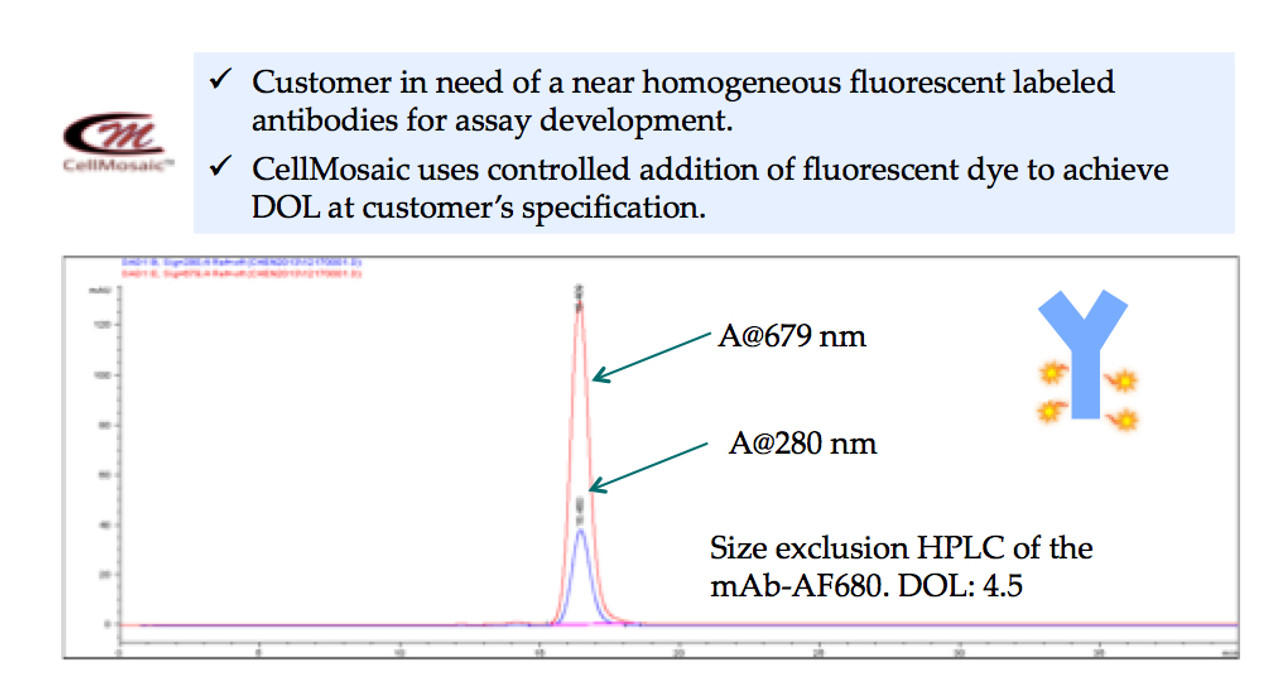
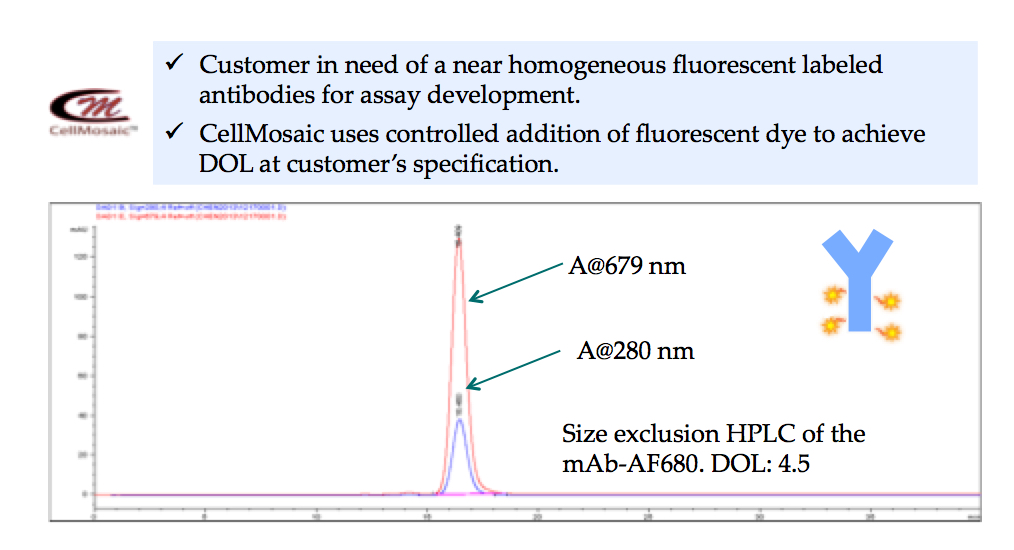
- Fluorescent labeling of a therapeutic antibody with defined DOL for assay development at CellMosaic® (shown below): over 99.9% pure by size exclusion HPLC.
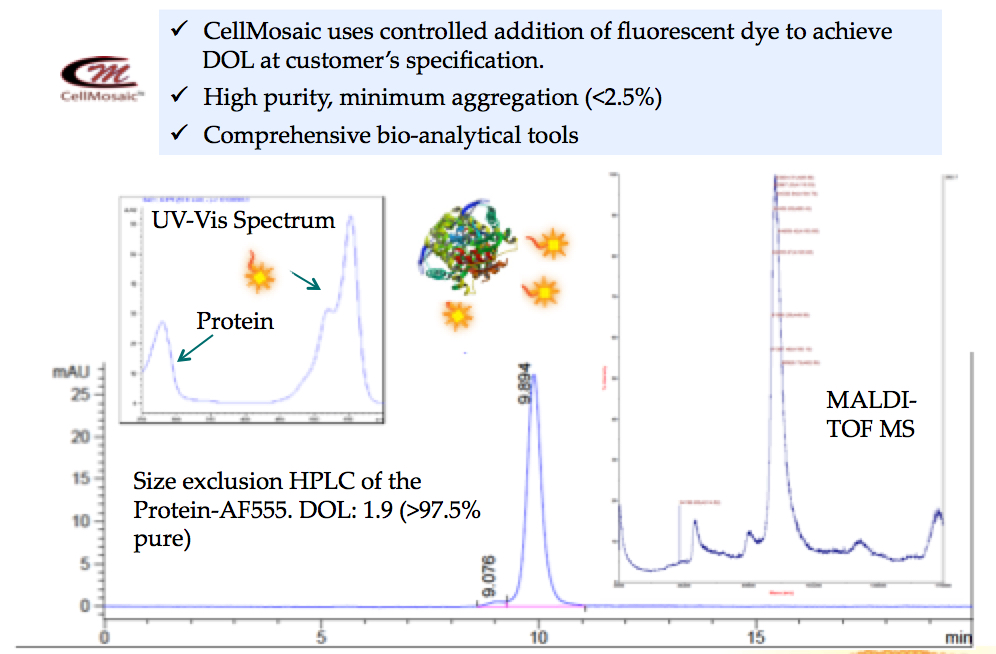
- Fluorescent labeling of a protein with defined DOL for assay development at CellMosaic® (shown below): over 97.5% pure by size exclusion HPLC, comprehensive bioanalytical tools were used to characterize the product.
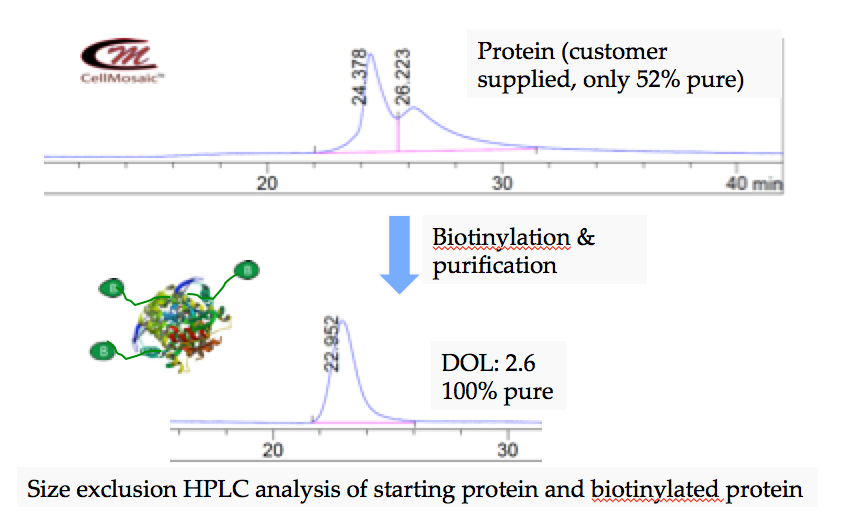
- Biotinylation of protein at CellMosaic® (shown below): only 52% pure starting protein was used and over 99.9% pure of biotinylated protein was isolated.
References:
- Jobbagy, A.; Kiraly K. Chemical characterization of fluorescein isothiocyanate-protein conjugates. Biochim Biophys Acta 1966 Jul 27;124(1):166-75. PMID: 4165223.
- Bragg, P.D.; Hou, C. Subunit composition, function, and spatial arrangement in the Ca2+-and Mg2+-activated adenosine triphosphatases of Escherichia coli and Salmonella typhimurium.Arch Biochem Biophys. 1975 Mar;167(1):311-21. PMID: 124154.
- Lomant, A. J.; Fairbanks, G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243-61.PMID: 957432.