Product Description
This product is a new formulation of our previously developed SEC protein standard (Part No. CM92002) and is a replacement for both SEC protein standards (Part No. CM92001 and CM92002).
| Download Documents | ||
| SDS |
Product Sheet | |
| COA-S216.S18.0715B |
||
| COA-S216.S18.1230D | ||
CellMosaic’s size exclusion chromatography (SEC) or gel filtration protein standard is designed for day to day use to check HPLC column performance and for analysis of bioconjugates. The new formulation allows the stable lyophilization of the protein mixture without changing its HPLC profile. The lyophilized protein mixture can be quickly reconstituted in water without any insoluble particles and is more stable for storage and shippment.
The kit consists of six different proteins and blue dextran with a MW range of 13.7 to 2000 KDa. The broad range of protein molecular weight coverage allows for easy identification of small molecules, conjugates, and aggregates in a bioconjugation mixture (antibody-drug conjugation, protein, and antibody modification and conjugation). This standard has been validated in many of the commonly used SEC columns and CellMosaic routinely uses this product for its internal bioconjugation-related research.
A total of four micro-centrifuge tubes are in a package. Each micro-centrifuge tube contains protein mixture lyophilized from 80 µL of standard solution. The product is ready for use after reconstitution in 80 µL of deionized water.
Shipping policy: heat-sensitive, choose overnight (ships Mon.-Thur.)
Application of the product:
-
Calibration of gel filtration and size exclusion column
-
Determination of the size and molecular mass of proteins
-
Analysis of small molecules, bioconjugates, and aggregates
Key Features of the product:
-
Lyophilized solid and ready to use for HPLC analysis after dissolving in water
-
Mixture of 6 high purity proteins with broad MW coverage: 13.7 KDa to 2000 KDa
SEC Standard Composition
| # | Name | MW (KDa) |
|---|---|---|
| 1 | RNase A | 13.7 |
| 2 | Carbonic anhydrase | 29 |
| 3 | Ovalbumin (albumin chicken egg) | 43 |
| 4 | Aldolase (rabbit muscle) | 158 |
| 5 | Ferritin (equine spleen) | 440 |
| 6 | Thyroglobulin | 669 |
| 7 | Blue Dextran | 2000 |
Application Note
Calibration of Protein and Conjugate MW
The approximate MW of an unknown protein or protein conjugate can be determined by comparing its retention time to the protein standard. Figure 1 gives an example of the SEC profile. For more accurate calculation, an SEC MW standard curve is recommended (Figure 2).
Figure 1: SEC HPLC profile of protein standards in a TSKgel BioAssist G3SWxl (7.8 mm x 300 mm, 5 µm, Part No. 0008541). HPLC condition: isocratic PBS buffer at 0.5 mL/min. Instrument: Agilent 1100 HPLC series.
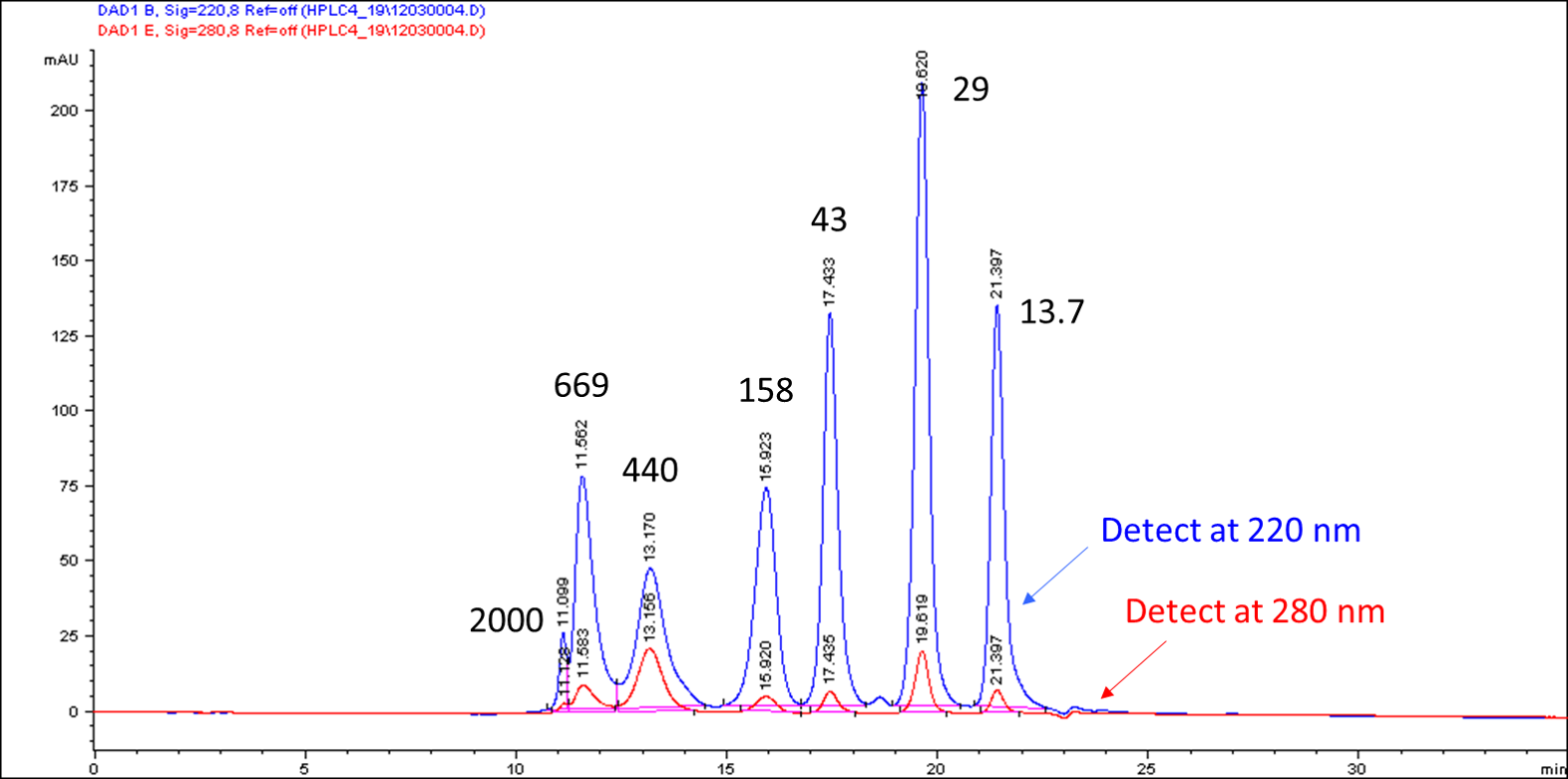
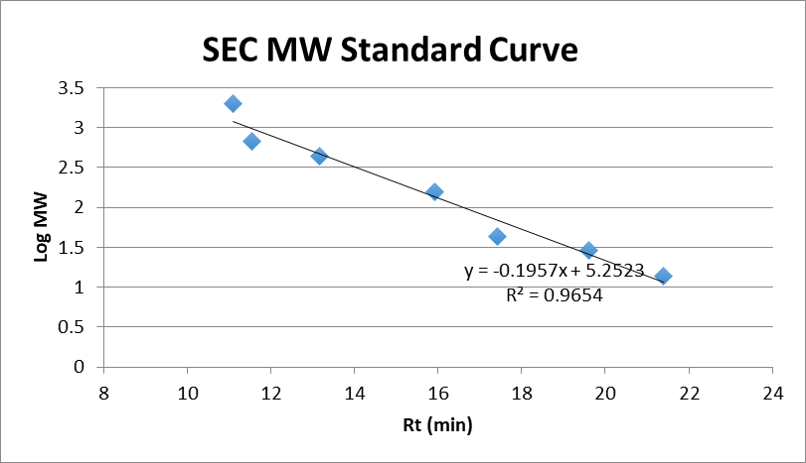
Figure 2: SEC MW standard curve of TSKgel BioAssist G3SWxl (7.8 mm x 300 mm, Part No. 0008541). Data from Figure 1.
Selected Citations from Customers who Use Our Product
Shon, H.; Matveeva, E. A.; Jull, E. C.; Hu, Y.; Coupet, T. A.; Lee, Y.-S. Evidence Supporting Substrate Channeling between Domains of Human PAICS: A Time-Course Analysis of 13C‑Bicarbonate Incorporation Biochemistry 2022, 61(7), 575-582 DOI: 10.1021/acs.biochem.1c00803
Collier, G.; Roovers A. L.; Hulleman, J. D. A spectrum of cysteine mutations in fibulin-3 highlight its disulfide bonding complexity and potential to induce ER stress Scientific Reports 2025, 15, 30028 DOI: 10.1038/s41598-025-14561-4
Azam, M.S.; Missiakas, D.; The signal peptide of staphylococcal protein A alters the multimeric states of PepV Microbiology Spectrum 2025, e01778-25 DOI: 10.1128/spectrum.01778-25
Frequently Asked Questions:
The list below includes FAQs that are specific to this product.
Question: How much total protein is in each vial of the SEC Protein Standard?
Answer: The products contain a mixture of proteins in a lyophilized format. Once you dissolve the product in 80 µL of deionized water, the concentration of each protein is within 0.01-1 mg/mL. There are approximately 1.5-3 mg of total protein content in one package (4x80 µL). We cannot supply the exact concentration of each protein because the product is designed for MW calibration and not for quantitation. If you need to perform a quantitation by HPLC, we suggest using BSA to create a standard curve.
Question: How do I improve the peak resolution and separation of the standard proteins for a particular column?
Answer: The separation in SEC is mainly determined by the resin/column you are using (particle size, modification, type). However, there are a few things you can do to improve the peak resolution and achieve better separation of the protein standards. For example, decrease the flow rate, decrease the injection loop size (not just the injection volume), shorten the line between the injector to the column, and clean the column before usage. The performance of the column decreases dramatically with use. Try to clean it and follow the manufacturer’s instructions before injecting the standard.
Question: Can I scale-up the recommended injection volume to match the length of my column?
Answer: Yes, the concentration of each indicator is optimized to use 8 μL for a standard SEC HPLC analytical column with a volume of 10-15 mL. If you have a larger volume column, you can scale the injection volume linearly. For example, if you have 200 mL resin, you may need to inject around 200 μL total (around 3 tubes, 4x80 μL provided). If you are using a loop size that accommodates significantly more volume than the volume you need to inject, you will need to inject enough volume so that when the standard dilutes into the loop volume it will still be around 0.1-1 mg/mL for each protein.
Question: Can I use CM92004 with an FPLC column (Cytiva column)?
Answer: Yes, you can use it with an FPLC column (Cytiva column). However, keep in mind that the peaks may not be as sharp as with the HPLC column shown in the product sheet, and the first two peaks (blue dextran and thyroglobulin) are not separable.
Question: Can I use CM92004 with a semi-preparative column?
Answer: For purification columns we generally suggest using our BSA standard for protein quantitation (CM92006), CM92004 is designed to be used in an analytical or gel filtration column. When used with columns packed using preparative resin, this standard will give broad, not well resolved, peaks and you will need to scale-up the volume of sample you are injecting (see Can I scale-up the recommended injection volume to match the length of my column?” for more information).
Question: Our sample is a 1200 KDa protein. Can I use CM92004 to generate a standard curve to calculate the MW?
Answer: On Page 4 of the CM92004 product sheet, there is a representative Log MW vs. Rt(min) graph (Figure 2). As you can see, Dextran is not very representative of proteins. To obtain reliable data, you can use data points from other proteins (or maybe only the high MW ones) to generate a graph for the calculation.
Question: I used CM92004 on a new Superdex 200 16/26 column (120 mL total volume). I injected 80 µL of the standard into a 2 mL loop, but I didn’t see any peak on the profile.
Answer: This SEC protein standard is designed for use in an analytical HPLC or gel filtration column. Gel filtration columns utilizing Superdex 200 typically produce slightly broader peaks, with peaks 1 and 2 often unresolved. A semi-preparative column (120 mL is being used here) requires a larger sample volume. Injecting 80 microliters of the sample into a 2 mL loop results in dilution within the loop before reaching the column, likely leading to minimal detection beyond a baseline increase. To use this standard effectively, it may be necessary to adjust the loop size to 100 µL or inject a larger sample volume at the same concentration, around 0.1-1 mg/mL for each protein. This approach could improve results. However, it should be noted that Superdex 200 preparative resin, with its large bead size, offers lower resolution for these proteins compared to standard HPLC SEC resin. For purification columns we commonly use BSA. A BSA standard (Cat# CM92006) is available for purchase if needed.
If you can’t find the answer you’re looking for or need information on general topics, please visit the main Frequently Asked Questions (FAQs)</st